Newsroom
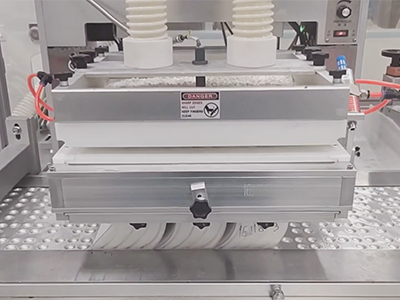
Blister Line equipped with a running Multiple-Tube Feed System.

Continuous blister line running at a European customer
10/01/2025
Preparation process and optimization of solid dosage forms
Solid dosage forms play a crucial role in the pharmaceutical industry, and the process and performance of their manufacturing equipment
09/06/2025
The Difference Between cGMP and GMP
1. What is cGMP?cGMP refers to current good manufacturing practices (GMP) regulations enforced by the FDA. cGMP provides a system
08/26/2025
A Blister Card Stacking and Arrangement Device
Blister packaging machines are key equipment for automated pharmaceutical packaging, particularly for solid dosage forms such as capsules and tablets.
08/02/2025
Solid Dosage Transport Validation
Solid Dosage transport validation is a crucial step in ensuring the stability of drug quality. It plays a vital role

The customers visit our factory.

The client came to visit the exhibition

Customer came to our trade show

Our partners came to visit the blister line

Have dinner with customers

07/11/2025
